Based on Hunterbrook Media’s reporting, Hunterbrook Capital is long $HIMS at the time of publication. Positions may change at any time. See full disclosures below.
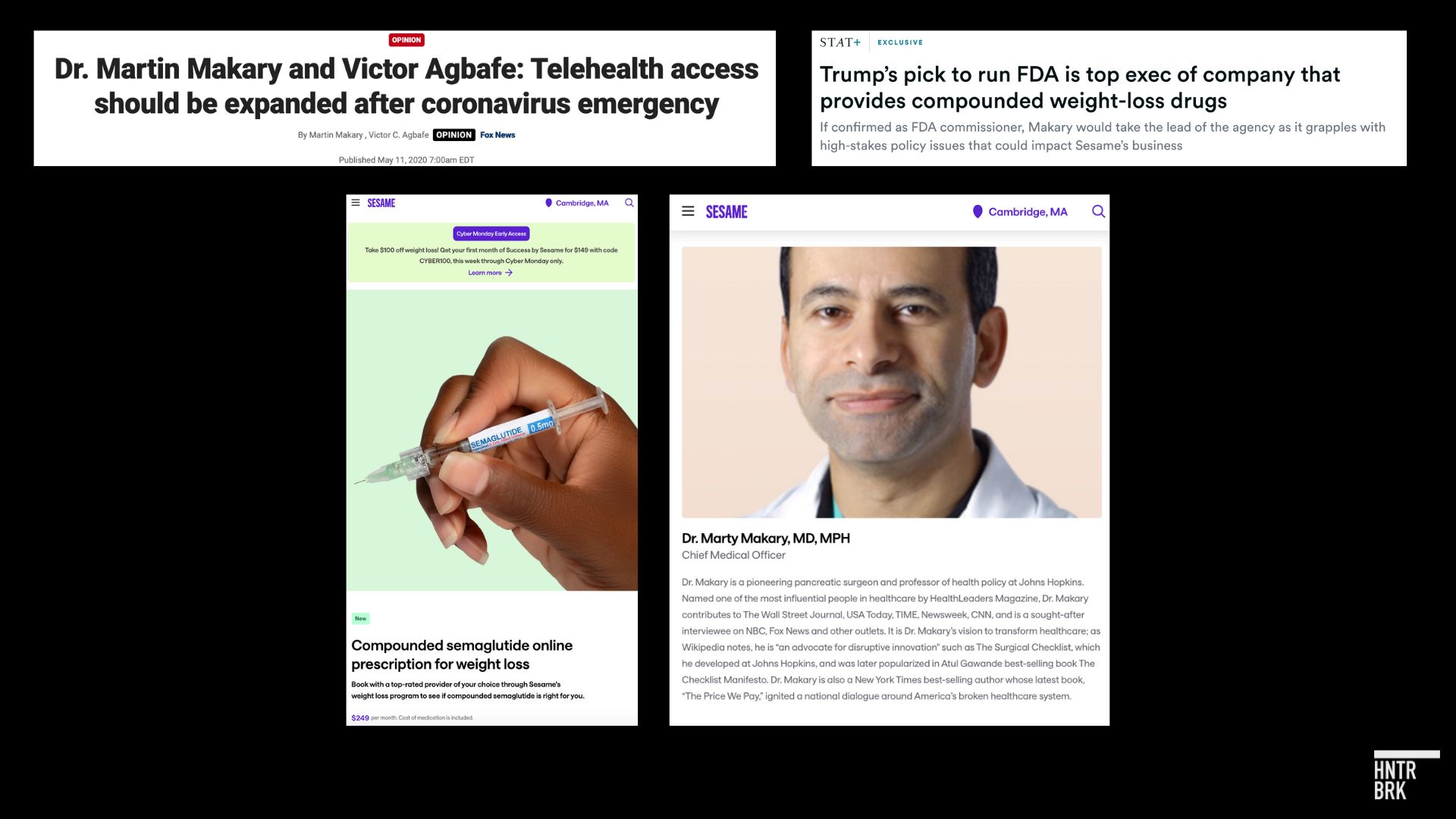
The FDA’s oversight of compounded GLP-1 drugs has complicated Hims & Hers Health Inc. ‘s (NYSE: $HIMS) foray into weight-loss drugs, but the telehealth giant may soon have a key ally in the Trump administration. STAT News reported on Sunday that President-elect Donald Trump has nominated Dr. Marty Makary, an executive of a startup called Sesame, which also sells compounded GLP-1 weight loss drugs online, to lead the Food and Drug Administration.
Hims — initially known for hair loss and sexual health products — has drawn scrutiny this year from medical experts for its rapid expansion into the weight-loss drug market. At the core of the controversy are GLP-1 drugs produced through a process called compounding, which means that the products are not FDA approved.
Compounded GLP-1 drugs are cheaper alternatives to the high-cost, FDA-approved, branded medications like Novo Nordisk’s Wegovy (semaglutide) and Eli Lilly’s Mounjaro (tirzepatide). Hims sells its version of these products under an FDA exemption that allows knockoff drugs to fill gaps during supply shortages, like the shortage of GLP-1 drugs amid extremely high demand.
The FDA’s erratic designation of those shortages has created a volatile regulatory environment. In October, the FDA removed tirzepatide from its shortage list, effectively barring compounding pharmacies from producing it. This catalyzed a lawsuit from the Outsourcing Facilities Association, representing compounding pharmacies, which argued that the decision was “reckless and arbitrary.” Just days later, the FDA reversed itself, reinstating tirzepatide’s shortage status and highlighting the uncertainty surrounding these regulatory decisions. Hims has said that it may attempt to continue providing compounded drugs through its 503B pharmacy even if the shortage ends.
“The FDA is in a flat spin,” said Dr. Rajesh Aggarwal, an obesity doctor and founding CEO of twenty30 health — describing the two-week period during which the FDA flip-flopped on this as “comical.”
“This is not how American officials should treat the American public.”
Aggarwal said he welcomes fellow GI surgeon Makary, whom he described as “evidence based and scientific in thought.” He said he believes Makary will be able to bring Lilly, Novo, and the compounders around the table to demand and drive clarity on the projected demand, medication supply, and pricing of GLP-1 meds.
Some of Makary’s past statements, particularly his advocacy of “natural immunity” during COVID-19, have received significant scrutiny.
For Hims, the uncertainty around the GLP-1 shortage has compounded other risks. Earlier this year, Hunterbrook Media exposed weaknesses in the company’s prescribing process for compounded GLP-1 drugs. The investigation revealed that patients could obtain prescriptions after brief online questionnaires, without speaking to a doctor. (Makary’s company, Sesame, appears to provide patients with a video consultation.) Hunterbrook also highlighted safety concerns with the supplier for Hims, BPI Labs, whose parent company has faced regulatory scrutiny and allegations of fraud.
Following Hunterbrook’s investigation, Hims updated its SEC risk disclosures, gave customers the opportunity to request certificates of analysis verifying that the compounded GLP-1 drugs have the appropriate ingredients, and — according to Wired — appears to have strengthened its prescribing requirements. Wired reported on July 12 that “Hims was the sole company that did not offer me GLP-1 meds, even though it accepted my exaggerated weight without verification,” as opposed to the earlier experience of a Hunterbrook reporter and sources interviewed for the investigation published on June 27.
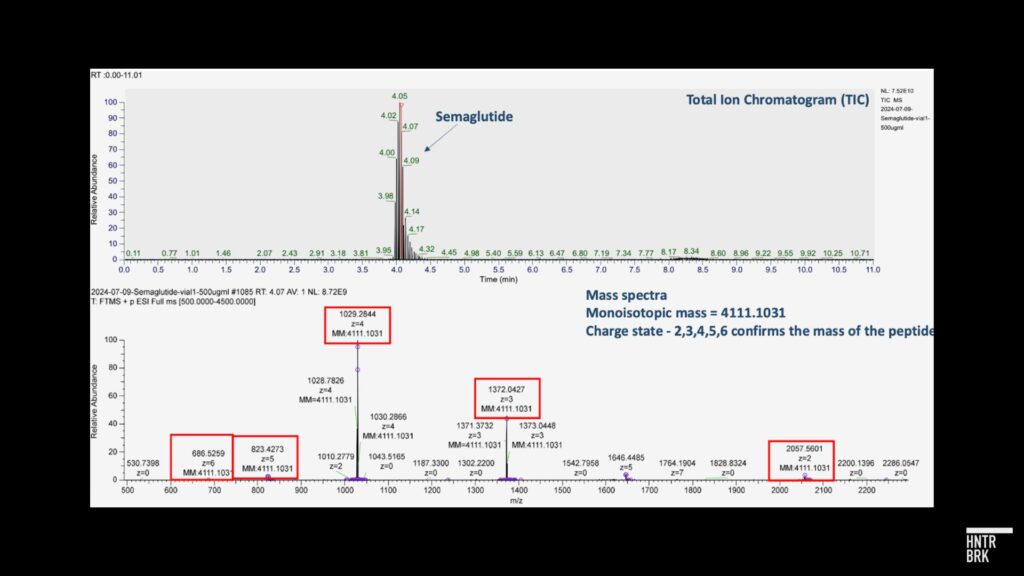
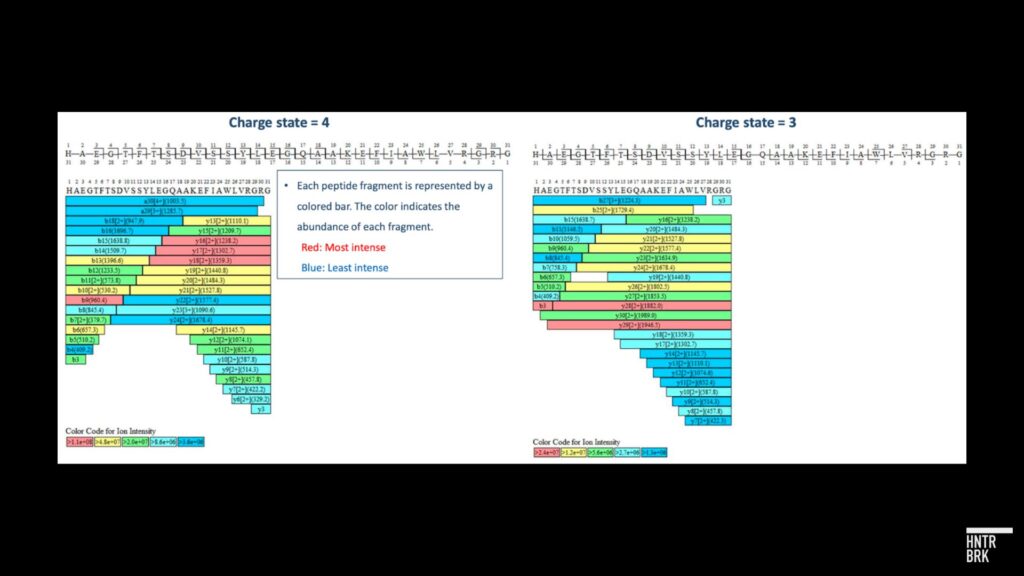
After its initial article, Hunterbrook analyzed samples acquired from Hims through an independent third-party laboratory that is FDA registered and inspected, GLP/cGMP compliant, and DEA licensed. The lab used common analytical chemistry techniques including peptide mapping via liquid chromatography-mass spectrometry (LC-MS) that confirmed that, unlike products sold by certain counterfeit GLP-1 providers, the tested samples were, in fact, semaglutide. A preliminary experiment using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) identified the presence of sodium in the tested samples. The lab reported to Hunterbrook: “Potential Reasons for Sodium Presence in the Sample: 1) Source of Semaglutide: The origin of the semaglutide is unknown, which could contribute to the presence of sodium, such as semaglutide sodium. 2) Formulation Buffer/Stabilizer/Excipient: The type of formulation buffer, stabilizer, or excipient used in the drug’s production may contain sodium. 3) Contamination: Sodium may be introduced through contamination during the manufacturing process. Based on the amount of sodium content measured, this is unlikely.” The lab noted that: “Further analyses are required to confirm the source of sodium measured in the samples provided.”
In response to a request for comment on Hunterbrook’s lab results, Hims shared that it had obtained “third-party testing” of its own, which also “confirmed the presence of the semaglutide base at the concentration reflected in the COA that we share with customers,” referring to the Certificates of Analysis that Hims made available to customers following Hunterbrook’s investigation.
The nomination of Makary may provide an opportunity for Hims to stabilize its business. Makary has experience with telehealth at Sesame and, as STAT News reported, previously sat on the board of a company called Harrow that prescribed “both compounded and brand-name eye drugs.”
The surgeon may not only be sympathetic to Hims on compounded semaglutide. In a 2020 Fox News op-ed, Makary made a full-throated case for “embracing telehealth,” arguing it expands “access to medical care and improves our nation’s health security.”
“It is time we revamp archaic and cumbersome patient services,” he wrote.
It’s a case of unlikely bedfellows: The Trump Administration providing a potential boost to a company whose CEO Andrew Dudum has spoken out in favor of Palestinian rights and donated more than $100,000 in the 2024 election cycle to progressive groups like the Justice Democrats and candidates like former Representatives Cory Bush and Jamaal Bowman, according to the FEC’s database. Dudum also supported the presidential campaign of Vice President Kamala Harris.
Makary’s confirmation would not erase all risks for Hims, of course. Big Pharma is aggressively litigating against compounding, and patient safety concerns remain.
It is also worth noting that Makary’s commentary on GLP-1 drugs has not been entirely positive.
“GLP-1 drugs could cost Medicare a fortune, diverting funds from other medical services,” he recently tweeted. And in his book, “Blind Spots,” he notes that while GLP-1 drugs “appear to be effective not only for losing weight but also in reducing the health problems associated with obesity such as heart disease, liver disease, and renal failure,” we “don’t yet know” the long-term consequences, citing “loss of muscle mass” as a risk.
“While it appears that we are seeing exciting benefits from these medications, we have to be open to the fact that future research could tell us that people on them long-term ultimately live longer, or shorter lives,” he writes.
Regardless, Makary may not be the decider on the FDA’s approach to GLP-1 compounding. As USC Professor Genevieve Kanter told STAT, FDA employees are required to recuse themselves from decisions related to former employers, which may impact Makary’s ability to weigh in on questions surrounding GLP-1 shortages.
In addition, as head of the FDA, Makary would be reporting to the Secretary of the Department of Health and Human Services — set to be Robert F. Kennedy Jr., if confirmed.
Kennedy has expressed skepticism about GLP-1 drugs as a panacea — and criticized their cost.
But hey, GLP-1s may grow on RFK: after all, mass-prescribing semaglutide through the mail may just be the best way to end the era of Big Seed Oil once and for all.
Author
Sam Koppelman is a New York Times best-selling author who has written books with former United States Attorney General Eric Holder and former United States Acting Solicitor General Neal Katyal. Sam has published in the New York Times, Washington Post, Boston Globe, Time Magazine, and other outlets — and occasionally volunteers on a fire speech for a good cause. He has a BA in Government from Harvard, where he was named a John Harvard Scholar and wrote op-eds like “Shut Down Harvard Football,” which he tells us were great for his social life. Sam is based in New York.
Editor
Jim Impoco is the award-winning former editor-in-chief of Newsweek who returned the publication to print in 2014. Before that, he was executive editor at Thomson Reuters Digital, Sunday Business Editor at The New York Times, and Assistant Managing Editor at Fortune. Jim, who started his journalism career as a Tokyo-based reporter for The Associated Press and U.S. News & World Report, has a Master’s in Chinese and Japanese History from the University of California at Berkeley.
Hunterbrook Media publishes investigative and global reporting — with no ads or paywalls. When articles do not include Material Non-Public Information (MNPI), or “insider info,” they may be provided to our affiliate Hunterbrook Capital, an investment firm which may take financial positions based on our reporting. Subscribe here. Learn more here.
Please contact ideas@hntrbrk.com to share ideas, talent@hntrbrk.com for work opportunities, and press@hntrbrk.com for media inquiries.
LEGAL DISCLAIMER
© 2026 by Hunterbrook Media LLC. When using this website, you acknowledge and accept that such usage is solely at your own discretion and risk. Hunterbrook Media LLC, along with any associated entities, shall not be held responsible for any direct or indirect damages resulting from the use of information provided in any Hunterbrook publications. It is crucial for you to conduct your own research and seek advice from qualified financial, legal, and tax professionals before making any investment decisions based on information obtained from Hunterbrook Media LLC. The content provided by Hunterbrook Media LLC does not constitute an offer to sell, nor a solicitation of an offer to purchase any securities. Furthermore, no securities shall be offered or sold in any jurisdiction where such activities would be contrary to the local securities laws.
Hunterbrook Media LLC is not a registered investment advisor in the United States or any other jurisdiction. We strive to ensure the accuracy and reliability of the information provided, drawing on sources believed to be trustworthy. Nevertheless, this information is provided "as is" without any guarantee of accuracy, timeliness, completeness, or usefulness for any particular purpose. Hunterbrook Media LLC does not guarantee the results obtained from the use of this information. All information presented are opinions based on our analyses and are subject to change without notice, and there is no commitment from Hunterbrook Media LLC to revise or update any information or opinions contained in any report or publication contained on this website. The above content, including all information and opinions presented, is intended solely for educational and information purposes only. Hunterbrook Media LLC authorizes the redistribution of these materials, in whole or in part, provided that such redistribution is for non-commercial, informational purposes only. Redistribution must include this notice and must not alter the materials. Any commercial use, alteration, or other forms of misuse of these materials are strictly prohibited without the express written approval of Hunterbrook Media LLC. Unauthorized use, alteration, or misuse of these materials may result in legal action to enforce our rights, including but not limited to seeking injunctive relief, damages, and any other remedies available under the law.