Based on Hunterbrook Media’s reporting, Hunterbrook Capital is short $DXCM at the time of publication. Positions may change at any time. Hunterbrook Law is working with the law firm Wisner Baum LLP, which filed a lawsuit against Dexcom. We’ve also petitioned the FDA to intervene and shared this investigation with a senior HHS official. Read the original investigation and accompanying disclosures here. See full disclosures below.
Three people with diabetes died following issues with Dexcom’s G7 continuous glucose monitor, according to adverse event reports filed in September with the FDA.
Dexcom “dropped the ball,” a recent Hunterbrook investigation found, with some diabetics who rely on the device to help manage their blood sugar ending up hospitalized — or dead.
After the investigation was published in September, the company’s stock dropped sharply. A pair of law firms then filed class action lawsuits against Dexcom, claiming its devices were not as accurate as advertised. A securities lawsuit was filed against the company last night.
Now, new reports of deaths have appeared on the FDA’s Manufacturer and User Facility Device Experience, or MAUDE, database. While anyone can submit a report to MAUDE on adverse events involving medical devices, manufacturers, importers, and healthcare facilities must notify the FDA when they learn of a serious problem involving a product.
These reports do not necessarily mean a device was responsible for the injury or death, though the database can help manufacturers and the FDA identify problematic products. MAUDE is updated monthly. Asked about these deaths, Dexcom — which is set to report earnings later this week — did not respond to repeated requests for comment.
At least 13 G7 users have died since its launch in 2023, according to MAUDE reports classified as deaths where the manufacturer was labeled as “Dexcom” and the brand name included “G7.” MAUDE data does not necessarily represent the full universe of deaths related to Dexcom devices. For example, we identified a reported death in MAUDE that was only specifically linked to the G7 in a follow-up report received 7/31; the death was first reported a week earlier, but that initial MAUDE submission did not specify the product model was the G7. Because “G7” was not mentioned in the first submission, the initial death report was not captured by our search parameters; Hunterbrook identified this initial report manually. There may be other reported deaths that Hunterbrook missed. The FDA sent Dexcom a warning letter in March 2025.
The latest MAUDE reports don’t reveal the names of those who died, but their stories reflect some common problems with the G7 identified by Hunterbrook’s previous reporting.
Do you know more about Dexcom or issues we should investigate? Reach us at ideas@hntrbrk.com.
A 69-year-old man allegedly died after an event on May 30, wearing a sensor that “was not showing any readings,” according to the MAUDE report. His wife reported he had “multiple” sensors that would last only one or two days before suffering connection issues. She claimed to have later gotten a letter from Dexcom saying his sensor “may have been part of a defective batch.”
A second person appears to have died after an event on August 30. She used the G7 in concert with an automated insulin pump, which uses G7 readings to deliver doses of the vital hormone. An inaccurate reading from the G7 can prompt an inaccurate dose of insulin.
She showed signs of ketoacidosis, which happens when someone’s blood sugar is too high. The day she suffered a fatal event, according to the report, the G7 showed a notably low blood sugar reading. When an ambulance arrived, she was given more glucose. She was taken to the ER, left alone for 20 minutes, then found unresponsive. When her blood sugar was finally checked, it was strikingly high and she was “in severe acidosis,” according to the report to the FDA. Emergency personnel tried for 45 minutes to resuscitate her, to no avail.
The report also noted that “the probable cause” of her death “could not be determined” and that “Dexcom has reached out to Tandem,” the maker of the insulin pump, “to request Tandem’s data investigation and CGM data.”
The third purported death was tied to the extremely low blood sugar of a 69 year-old woman who depended on the G7’s receiver to provide alerts, according to a report to the FDA. The event happened May 18, six days after Dexcom publicly announced a recall for receivers that didn’t sound alarms as expected. The report notes that, after her death, “a letter from dexcom was received” about the alarm defect. The report was a follow-up; her death was initially reported August 12.
“I’m ending up in the ER from a device that’s supposed to save my life but now it seems like it’s slowly killing me.”
— Emily Carnes, ICU nurse Hospitalized After G7 “Faulted”
These stories align with conversations Hunterbrook has had with endocrinologists across the country, who have reported repeated G7 failures. Two said when they turned to the company for answers, representatives acted surprised, which one doctor described as “gaslighting.” Hunterbrook spoke to other G7 users who also described inaccurate sensor readings leading to crises.
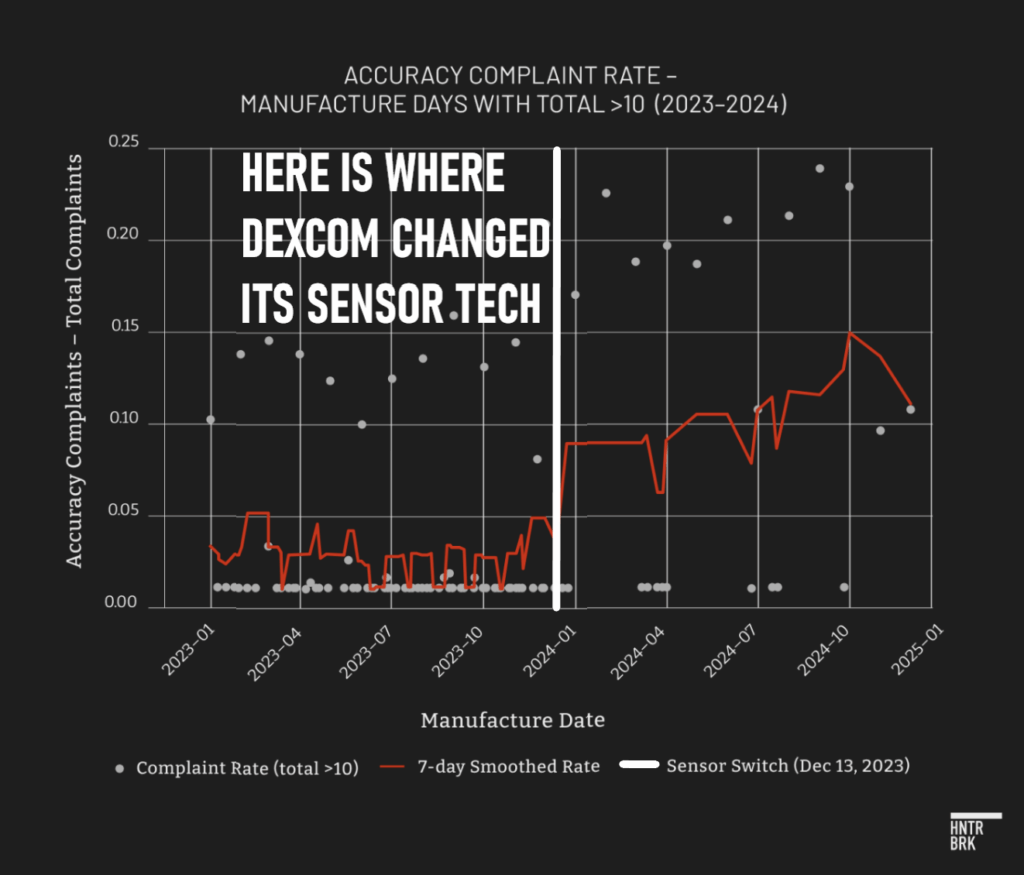
The day the Hunterbrook investigation was published — revealing a spike in G7 accuracy-related complaints after Dexcom made a manufacturing change — Emily Carnes, an ICU nurse, ended up in the emergency room as a patient.
The G7 had been her “lifeline,” she told Hunterbrook. She relied upon it every hour of the day to dose her insulin and keep her alive. She loved Dexcom’s previous G6 and was excited about the faster and smaller G7.
She was on a work trip and started her day normally. Coffee with creamer, then a check of her G7 blood sugar reading. When she got down to her hotel lobby, her G7 alerted her that her blood sugar was 115.
That’s a bit high, but she felt “dazed and confused.” So she trusted her instinct, chugged juice, and pricked her finger. Her blood sugar was actually 33 — dangerously low.
“I fell down,” she said.
She hit her head and spent four to five hours in the emergency room.
“I’m ending up in the ER from a device that’s supposed to save my life but now it seems like it’s slowly killing me,” Carnes said.
What was once a lifeline had become life-threatening.
“It’s all because the G7 faulted.”
Authors
Andrew Ford is an investigative journalist who has exposed systemic flaws and prompted reforms in healthcare, business, policing and state government. His reporting has been published by ProPublica, USA Today, The Arizona Republic, Asbury Park Press, and Florida Today. He holds a journalism bachelor’s from the University of Florida and is in his final semester of a data analytics master’s from Georgia Institute of Technology. He’s based in Phoenix, AZ.
Laura Wadsten is an investigative journalist specializing in healthcare. She began her career reporting on antitrust and health care markets as a Correspondent for The Capitol Forum, a premium subscription financial publication. Previously, she was Executive Director of the nonprofit Moving to Value Alliance, where she produced the MTVA Unscripted Podcast. Laura was a Hodson Scholar and Editor-in-Chief of The News-Letter at Johns Hopkins University, where she earned a B.A. in Medicine, Science & the Humanities.
Michelle Cera trained as a sociologist specializing in digital ethnography and pedagogy. She completed her PhD in Sociology at New York University, building on her Bachelor of Arts degree with Highest Honors from the University of California, Berkeley. She has also served as a Workshop Coordinator at NYU’s Anthropology and Sociology Departments, fostering interdisciplinary collaboration and innovative research methodologies.
Editors
Jim Impoco is the award-winning former editor-in-chief of Newsweek. Before that, he was executive editor at Thomson Reuters Digital, Sunday Business Editor at The New York Times, and Assistant Managing Editor at Fortune. Jim, who started his journalism career as a Tokyo-based reporter for The Associated Press and U.S. News & World Report, has a Master’s in Chinese and Japanese History from the University of California at Berkeley.
Sam Koppelman is a New York Times best-selling author who has written books with former United States Attorney General Eric Holder and former United States Acting Solicitor General Neal Katyal. Sam has published in the New York Times, Washington Post, Boston Globe, Time Magazine, and other outlets. He has a BA in Government from Harvard, where he was named a John Harvard Scholar and wrote op-eds like “Shut Down Harvard Football,” which he tells us were great for his social life.
Hunterbrook Media publishes investigative and global reporting — with no ads or paywalls. When articles do not include Material Non-Public Information (MNPI), or “insider info,” they may be provided to our affiliate Hunterbrook Capital, an investment firm which may take financial positions based on our reporting. Subscribe here. Learn more here.
Please email ideas@hntrbrk.com to share ideas, talent@hntrbrk.com for work, or press@hntrbrk.com for media inquiries.
LEGAL DISCLAIMER
© 2026 by Hunterbrook Media LLC. When using this website, you acknowledge and accept that such usage is solely at your own discretion and risk. Hunterbrook Media LLC, along with any associated entities, shall not be held responsible for any direct or indirect damages resulting from the use of information provided in any Hunterbrook publications. It is crucial for you to conduct your own research and seek advice from qualified financial, legal, and tax professionals before making any investment decisions based on information obtained from Hunterbrook Media LLC. The content provided by Hunterbrook Media LLC does not constitute an offer to sell, nor a solicitation of an offer to purchase any securities. Furthermore, no securities shall be offered or sold in any jurisdiction where such activities would be contrary to the local securities laws.
Hunterbrook Media LLC is not a registered investment advisor in the United States or any other jurisdiction. We strive to ensure the accuracy and reliability of the information provided, drawing on sources believed to be trustworthy. Nevertheless, this information is provided "as is" without any guarantee of accuracy, timeliness, completeness, or usefulness for any particular purpose. Hunterbrook Media LLC does not guarantee the results obtained from the use of this information. All information presented are opinions based on our analyses and are subject to change without notice, and there is no commitment from Hunterbrook Media LLC to revise or update any information or opinions contained in any report or publication contained on this website. The above content, including all information and opinions presented, is intended solely for educational and information purposes only. Hunterbrook Media LLC authorizes the redistribution of these materials, in whole or in part, provided that such redistribution is for non-commercial, informational purposes only. Redistribution must include this notice and must not alter the materials. Any commercial use, alteration, or other forms of misuse of these materials are strictly prohibited without the express written approval of Hunterbrook Media LLC. Unauthorized use, alteration, or misuse of these materials may result in legal action to enforce our rights, including but not limited to seeking injunctive relief, damages, and any other remedies available under the law.